Cancer Genomics

BRCA1/2 Gene Mutation Testing
BRCA Gene and Breast Cancer
BRCA1 and BRCA2 are tumor suppressor genes that help repair damaged DNA and prevent uncontrolled cell growth. Mutations in these genes increase the risk of breast and ovarian cancer significantly. Women with a BRCA1 or BRCA2 mutation have up to a 72% lifetime risk of developing breast cancer and up to 44% risk of ovarian cancer. Men with BRCA mutations also have an increased risk of male breast and prostate cancer.

Who Should Get Tested for BRCA1 & BRCA2?
Individuals with a personal or family history (1st, 2nd, or 3rd-degree relatives) of cancers associated with BRCA1 and BRCA2 mutations may have a higher risk of Hereditary Breast and Ovarian Cancer (HBOC). Consider testing if you have:
A Personal history of :-
- Breast or ovarian cancer diagnosed at a young age (premenopausal)
- Bilateral breast cancer (affecting both breasts)
- Both breast and ovarian cancer
A Family history of :-
- Breast, ovarian, fallopian tube, peritoneal, prostate, or pancreatic cancer
- A male relative diagnosed with breast cancer
- A known harmful BRCA1 or BRCA2 gene mutation in the family
- Breast or ovarian cancer diagnosed before the age of 45
- Bilateral breast cancer in a family member below the age of 50
- Triple-negative breast cancer diagnosed below the age of 60, with or without a family history
- Breast and ovarian cancer in the same woman or family

Why Should You Get Tested for BRCA1 & BRCA2?
Specific inherited mutations in the BRCA1 and BRCA2 genes significantly increase the risk of developing breast and ovarian cancer.
BRCA gene testing helps with:
- Assessing hereditary cancer risk to understand your genetic predisposition.
- Guiding treatment decisions by helping clinicians recommend preventive measures.
- Targeted therapy planning for individuals already diagnosed with cancer, enabling the use of PARP inhibitors.
- Enhancing survival rates through early detection and timely intervention.
- Approximately 1 in 500 women in India carry a BRCA1 or BRCA2 mutation.
- BRCA mutations account for around 25% of hereditary breast and ovarian cancers in India.
- The incidence of breast cancer in Indian women has increased by 39.1% over the past two decades, with genetic factors playing a significant role.
Somatic
Somatic Mutation Testing: Detects BRCA mutations acquired in tumor cells, which can help guide targeted therapy and treatment decisions

Germline
Germline Mutation Testing: Identifies inherited BRCA mutations passed down through families and helps determine the hereditary risk of cancer.
FAQ Frequently Asked Questions
- Individuals with a personal or family history of breast, ovarian, prostate, or pancreatic cancer should consider testing.
- A positive result indicates a higher risk of developing certain cancers but does not mean cancer is inevitable.
- Yes, men can inherit and pass on BRCA mutations and have an increased risk of prostate and male breast cancer.
- Some insurance providers may cover genetic testing; it is advisable to check with your insurance company.
- Options include increased surveillance, lifestyle modifications, preventive surgery, or chemoprevention under medical guidance.
Your Genes tell a story - Make sure it’s one of Empowerment & Health
with our advanced Cancer Genomics tests You’re not just detecting disease you’re taking change of your Future

Whole Exome Sequencing (WES)
Whole Exome Sequencing (WES) is a comprehensive genetic test that examines the exome, which contains the coding regions of approximately 20,000 genes. Most of the genetic variants responsible for genetic diseases are found in the exome, making WES a powerful tool for identifying potential genetic causes of medical conditions. Unlike traditional genetic tests that analyze a single gene or a small set of genes, WES evaluates all coding regions in a single test using advanced next-generation sequencing (NGS) technology.
When is WES Recommended?
Whole Exome Sequencing is recommended at any stage of life—pregnancy, childhood, or adulthood—when:
- A genetic condition is suspected.
- Symptoms are complex and may be caused by multiple genes.
- There is a complicated medical history affecting multiple organs or systems.
- Previous genetic tests have yielded inconclusive results.

Indications for Testing:
WES is particularly beneficial for patients with:
- Broad, complex, or unspecific symptoms, such as developmental delay, intellectual disability, autism spectrum disorder, or epilepsy.
- Suspected chromosomal imbalances or syndromes, including microdeletions and microduplications, such as DiGeorge syndrome.
- Clinical suspicion of mitochondrial disease, presenting with muscular weakness, cardiomyopathy, or visual problems.
- Severe neonatal or childhood conditions, often seen in critically ill neonates or pediatric patients in NICU and PICU.
- Unresolved genetic conditions, where previous microarray or genetic tests have been inconclusive.
Cancer whole exome sequencing provides crucial insights into genetic mutations that contribute to tumor development and progression. Since the exome represents less than 2% of the genome, WES is a cost-effective and data-efficient alternative to whole genome sequencing (WGS). This test helps identify actionable mutations for targeted therapy, prognosis, and personalized treatment plans in oncology.
images/services/What is Covered in Exome Sequencing.png
WES analyzes:
- All protein-coding regions of the genome (exons).
- Single nucleotide variants (SNVs) and small insertions/deletions.
- Copy number variations (CNVs).
- Structural rearrangements.
- Mitochondrial genome (optional add-on).

FAQ Frequently Asked Questions
- Individuals with complex, undiagnosed medical conditions, or those with a family history of genetic disorders should consider WES.
- A DNA sample is collected from blood or saliva and analyzed using NGS technology to identify genetic variations.
- Results are typically available within 4-6 weeks, depending on the complexity of the analysis.
- Yes, all genetic information is kept confidential and shared only with authorized healthcare providers.
- While WES is a powerful diagnostic tool, it may not detect non-coding or regulatory region mutations that require whole genome sequencing.

Karyotyping:
Karyotyping is a genetic test that examines chromosomal abnormalities in cancer cells. One of the most commonly used techniques in cancer diagnostics is GTG (G-bands by Trypsin using Giemsa) banding.
GTG banding is crucial in identifying hematological malignancies and solid tumors by detecting chromosomal aberrations such as:
Leukemia & Lymphomas:
- Philadelphia Chromosome (t(9;22)) in CML
- t(8;14) in Burkitt's Lymphoma
- t(15;17) in Acute Promyelocytic Leukemia (APL)
Multiple Myeloma:
- Chromosome 13q deletion and t(11;14) translocation
Solid Tumors:
- Structural changes in breast cancer, sarcomas, and neuroblastomas
GTG banding remains a gold-standard technique in cancer cytogenetics, helping oncologists make informed decisions about treatment strategies.
Comprehensive Genome Profiling & TMB
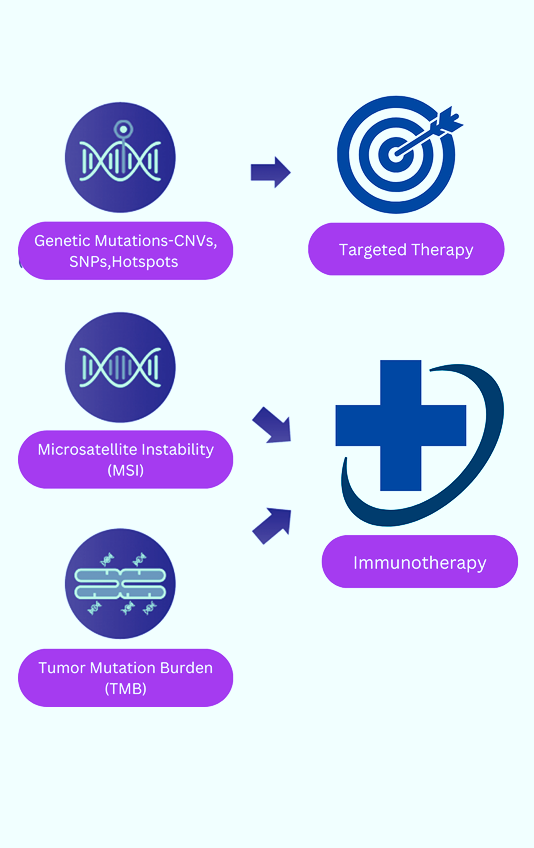
Comprehensive Genomic Profiling (CGP) is an advanced next-generation sequencing (NGS) test that examines hundreds of genes in a single assay, providing a detailed molecular profile of a tumor. It detects key cancer-related biomarkers and all major genomic alterations, including single nucleotide variants (SNVs), indels, copy number variants (CNVs), fusions, and splice variants. Additionally, CGP identifies genomic signatures such as tumor mutational burden (TMB) and microsatellite instability (MSI), which are critical for guiding personalized cancer treatment.
Who Should Consider CGP Testing?
CGP is recommended for:
- Patients with advanced, metastatic, or recurrent cancers where treatment decisions depend on genetic mutations.
- Individuals with rare or aggressive cancers that lack standard treatment options.
- Patients who have not responded to standard therapy and need alternative treatment strategies.
- Those with a family history of cancer who may benefit from targeted or preventive strategies.
- Oncologists seeking to enroll patients in clinical trials based on specific genetic mutations.
Prognosis, and treatment selection, ensuring that patients receive the most effective, personalized therapies.
Why Choose CGP for TMB Testing?
Traditional single-gene or small hotspot panel testing methods often miss key biomarkers, limiting the ability to detect clinically actionable alterations. CGP overcomes these limitations by:
- Offering nucleotide-level resolution for accurate biomarker detection.>
- Capturing large genomic signatures, including TMB and MSI (Microsatellite Instability).
- Providing a comprehensive molecular profile, covering known and emerging biomarkers in a single test.
How is the Test Performed?
CGP is conducted using a small tissue biopsy or a blood sample (liquid biopsy). The sample is analyzed with NGS technology, and results are reviewed by experts to provide actionable insights for treatment planning.
Hereditary Cancer Panel
Uncover Your Genetic Risk for Cancer
Hereditary cancer syndromes account for approximately 5%–10% of all cancers. If multiple family members on the same side have the same or related cancers, cancer occurs at an early age, or an individual has multiple cancers, a hereditary cancer risk may be suspected. Identifying these risks through genetic testing can aid in early detection, prevention, and personalized treatment strategies.
What Is the Hereditary Cancer Panel?
Our Hereditary Cancer Panel is a comprehensive genetic test that analyzes multiple genes associated with inherited cancer syndromes, including:
- Hereditary Breast and Ovarian Cancer (HBOC) Syndrome
- Lynch Syndrome (Hereditary Non-Polyposis Colorectal Cancer - HNPCC)
- Li-Fraumeni Syndrome
- Cowden Syndrome
- Familial Adenomatous Polyposis (FAP)
- Von-Hippel Lindau (VHL) Syndrome
- Multiple Endocrine Neoplasia (MEN) Type 1 & 2
Most hereditary cancer syndromes follow an autosomal dominant pattern of inheritance, meaning a single altered copy of a gene can increase cancer risk significantly.
Who Should Consider Testing?
Genetic testing for hereditary cancer syndromes is recommended if you have:
- A family history of the same or related cancers (breast, ovarian, colon, pancreatic, etc.).
- A personal or family history of cancer diagnosed at an early age (typically before 50).
- Multiple cancers in a single individual.
- Rare cancers such as male breast cancer or medullary thyroid cancer.
- Ashkenazi Jewish ancestry, which is associated with a higher prevalence of BRCA mutations.

Comprehensive Solid Tumor Panel
The Comprehensive Solid Tumor Panel is a cutting-edge genetic test designed to analyze genetic alterations in solid tumors such as lung, breast, colorectal, and other cancers. By leveraging next-generation sequencing (NGS) technology, this test provides a detailed molecular profile of the tumor, allowing for personalized treatment strategies and better patient outcomes.
Why Is the Solid Tumor Panel Essential?
This panel examines multiple genetic alterations that contribute to cancer development and progression, including:
Hotspot Mutations – Specific, frequently altered genetic regions linked to cancer.
Coding Sequence (CDS) Alterations – Mutations affecting gene function.
Copy Number Variations (CNV) – Changes in gene copy numbers leading to tumor growth.
RNA Fusions – Abnormal gene fusions driving cancer progression.
Prognostic & Predictive Value
Predicts tumor aggressiveness – Helps assess cancer severity and potential progression.
Guides treatment planning – Provides insights into the likelihood of response to different therapies.
Monitors treatment response – Enables real-time tracking of therapeutic effectiveness.
Microsatellite Instability (MSI) testing:
Microsatellite Instability (MSI) Testing is a genetic test used to detect changes in short, repetitive DNA sequences (microsatellites) due to defects in the DNA mismatch repair (MMR) system. It plays a crucial role in diagnosing certain cancers and guiding treatment decisions.
When Is MSI Testing Recommended?
- Patients with colorectal cancer (CRC), especially those diagnosed at a young age or with a family history.
- To assess for Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer - HNPCC), a genetic condition that increases cancer risk.
- In some cases, MSI testing is done for gastric, ovarian, pancreatic, and prostate cancers.
- MSI-High (MSI-H) tumors respond well to immune checkpoint inhibitors like pembrolizumab (Keytruda).
- Helps oncologists determine if a patient is a candidate for immunotherapy.
What Does the Test Result Mean?
- Chromosome 13q deletion and t(11;14) translocation
Solid Tumors:
- MSI-High (MSI-H): Indicates high genetic instability, often linked to Lynch syndrome or a better response to immunotherapy.
- MSI-Low (MSI-L) / MSS (Microsatellite Stable): Suggests a lower likelihood of Lynch syndrome and different treatment strategies.
